Предмет: Химия,
автор: kakashka41
Помогите пж очень срочно!!!!!!! Даю 15 баллов
Приложения:
Ответы
Автор ответа:
1
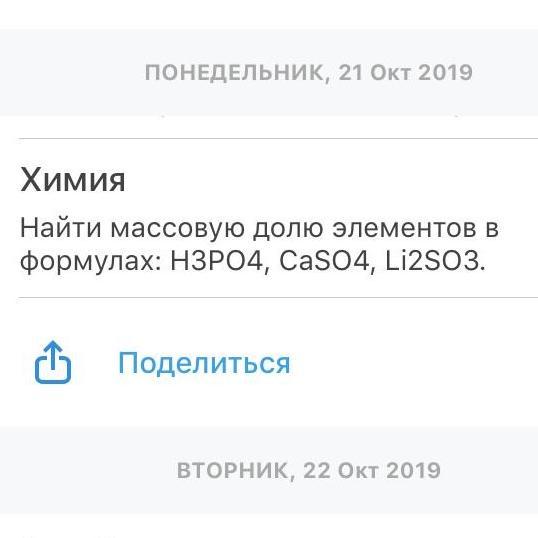
1) Mr(H3PO4) = 1*3 + 31 + 16*4 = 3 + 31 + 64 = 98 г/моль
W(H) = (3/98)*100% = 3%
W(P) = (31/98)*100% = 31.7%
W(O) = 100 - 3.06 - 31.6 = 65.3%
2) Mr(CaSO4) = 40+ 32 + 16*4= 136 г/моль
w(Ca) = 40 / 136 * 100% = 29,41%
w(S) = 32/ 136 *100% = 23,53%
w(O) = 16*4 / 136 = 47,06%
3) Mr(Li2CO3) = 7*2+12+16*3 = 74
w(Li) = 7*2/74 = 19%
w(C) = 12/74 = 16,2%
w(O) = 16*3/74 = 64,8%
W(H) = (3/98)*100% = 3%
W(P) = (31/98)*100% = 31.7%
W(O) = 100 - 3.06 - 31.6 = 65.3%
2) Mr(CaSO4) = 40+ 32 + 16*4= 136 г/моль
w(Ca) = 40 / 136 * 100% = 29,41%
w(S) = 32/ 136 *100% = 23,53%
w(O) = 16*4 / 136 = 47,06%
3) Mr(Li2CO3) = 7*2+12+16*3 = 74
w(Li) = 7*2/74 = 19%
w(C) = 12/74 = 16,2%
w(O) = 16*3/74 = 64,8%
Похожие вопросы
Предмет: Алгебра,
автор: 2krubwinxa
Предмет: Геометрия,
автор: nastia2492
Предмет: Психология,
автор: lelsdin21
Предмет: Химия,
автор: fjutkivmkf1023
Предмет: Алгебра,
автор: Jelaya