срочнооо даю 70 баллов
Ответы
Ответ:
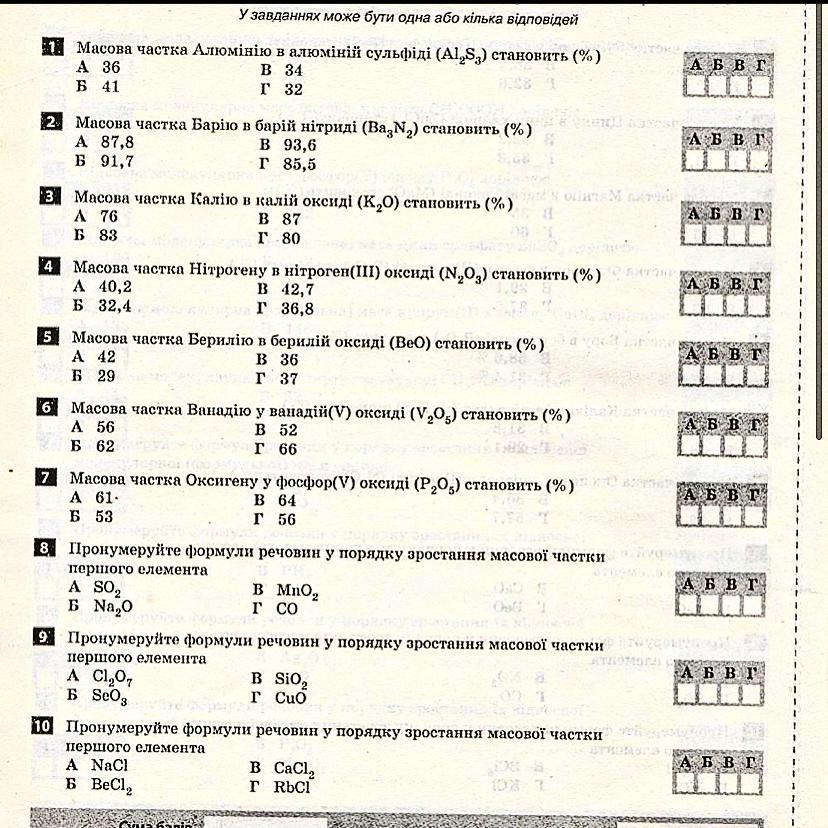
1 - а
Mr[Al2S3] = Ar[Al] * 2 + Ar[S] * 3 = 27 * 2 + 32 * 3 = 150
ω (Al) = 54 : 150 * 100 = 36 %
ω (S) = 96 : 150 * 100 = 64 %
2 - в
Mr[Ba3N2] = Ar[Ba] * 3 + Ar[N] * 2 = 137 * 3 + 14 * 2 = 440
ω (Ba) = 412 : 440 * 100 = 94 %
ω (N) = 28 : 440 * 100 = 6 %
3 - б
Mr[K2O] = Ar[K] * 2 + Ar[O] = 39 * 2 + 16 = 94
ω (K) = 78 : 94 * 100 = 83 %
ω (O) = 16 : 94 * 100 = 17 %
4 - г
Mr[N2O3] = Ar[N] * 2 + Ar[O] * 3 = 14 * 2 + 16 * 3 = 76
ω (N) = 28 : 76 * 100 = 37 %
ω (O) = 48 : 76 * 100 = 63 %
5 - в
Mr[BeO] = Ar[Be] + Ar[O] = 9 + 16 = 25
ω (Be) = 9 : 25 * 100 = 36 %
ω (O) = 16 : 25 * 100 = 64 %
6 - а
Mr[V2O5] = Ar[O] * 5 + Ar[V] * 2 = 16 * 5 + 51 * 2 = 182
ω (O) = 80 : 182 * 100 = 44 %
ω (V) = 102 : 182 * 100 = 56 %
7 - г
Mr[P2O5] = Ar[P] * 2 + Ar[O] * 5 = 31 * 2 + 16 * 5 = 142
ω (P) = 62 : 142 * 100 = 44 %
ω (O) = 80 : 142 * 100 = 56 %
8. Na2O, MnO2, SO2, CO ⇒ б, в, а, г.
Mr[SO2] = Ar[O] * 2 + Ar[S] = 16 * 2 + 32 = 64
ω (O) = 32 : 64 * 100 = 50 %
ω (S) = 32 : 64 * 100 = 50 %
Mr[Na2O] = Ar[Na] * 2 + Ar[O] = 23 * 2 + 16 = 62
ω (Na) = 46 : 62 * 100 = 74 %
ω (O) = 16 : 62 * 100 = 26 %
Mr[MnO2] = Ar[Mn] + Ar[O] * 2 = 55 + 16 * 2 = 87
ω (Mn) = 55 : 87 * 100 = 63 %
ω (O) = 32 : 87 * 100 = 37 %
Mr[CO] = Ar[C] + Ar[O] = 12 + 16 = 28
ω (C) = 12 : 28 * 100 = 43 %
ω (O) = 16 : 28 * 100 = 57 %
9. CuO, SeO3, SiO2, Cl2O7 ⇒ г, б, в, а.
Mr[Cl2O7] = Ar[Cl] * 2 + Ar[O] * 7 = 35 * 2 + 16 * 7 = 183
ω (Cl) = 71 : 183 * 100 = 39 %
ω (O) = 112 : 183 * 100 = 61 %
Mr[SeO3] = Ar[O] * 3 + Ar[Se] = 16 * 3 + 79 = 127
ω (O) = 48 : 127 * 100 = 38 %
ω (Se) = 79 : 127 * 100 = 62 %
Mr[SiO2] = Ar[O] * 2 + Ar[Si] = 16 * 2 + 28 = 60
ω (O) = 32 : 60.0843 * 100 = 53%
ω (Si) = 28 : 60 * 100 = 47 %
Mr[CuO] = Ar[Cu] + Ar[O] = 64 + 16 = 80
ω (Cu) = 64 : 80 * 100 = 80 %
ω (O) = 16 : 80 * 100 = 20 %
10. RbCl, NaCl, CaCl2, BeCl2 ⇒ г, а, в, б
Mr[NaCl] = Ar[Cl] + Ar[Na] = 35 + 23 = 58
ω (Cl) = 35 : 58 * 100 = 61 %
ω (Na) = 23 : 58 * 100 = 39 %
Mr[BeCl2] = Ar[Be] + Ar[Cl] * 2 = 9 + 35 * 2 = 80
ω (Be) = 9 : 80 * 100 = 11 %
ω (Cl) = 71 : 80 * 100 = 89 %
Mr[CaCl2] = Ar[Ca] + Ar[Cl] * 2 = 40 + 35 * 2 = 111
ω (Ca) = 40 : 111 * 100 = 36 %
ω (Cl) = 71 : 111 * 100 = 64 %
Mr[RbCl] = Ar[Rb] + Ar[Cl] = 85 + 35 = 121
ω (Rb) = 85 : 121 * 100 = 71 %
ω (Cl) = 35 : 121 * 100 = 29 %
Объяснение: