Предмет: Химия,
автор: Bangtansonentan
ПОМОГИТЕ ПО ХИМИИ СРОЧНО!! 50 БАЛЛОВ!
Приложения:
Ответы
Автор ответа:
0
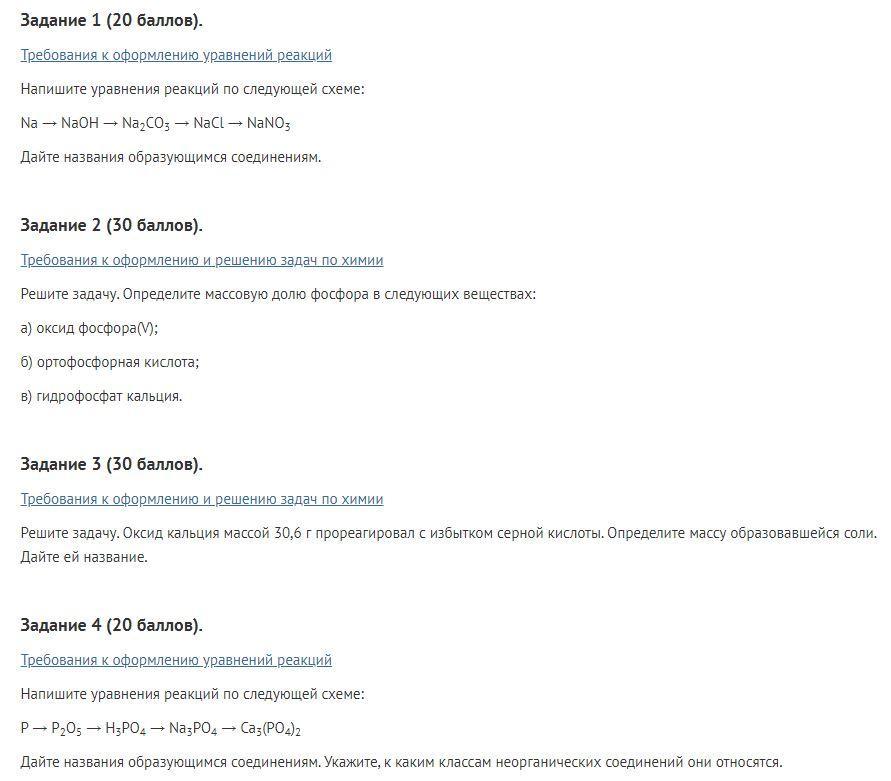
Задание 1
2Na + 2H2O —> 2NaOH + H2 ( гидроксид натрия и водород )
2NaOH + H2CO3 —> Na2CO3 + 2H2O
( карбонат натрия и вода )
Na2CO3 + 2HCl —> 2NaCl + H2O + CO2
( хлорид натрия и карбонатная кислота, которая сразу распадается на воду и углекислый газ )
NaCl + HNO3 —> NaNO3 + HCl ( нитрат натрия и соляная кислота )
Задание 2
Для вычисления массовой доли фосфора используем формулу:
W ( элемента ) = n ( количество атомов элемента ) • Ar ( атомную массу элемента )
и делим это на Mr ( молекулярную массу вещества )
1) массовая доля фосфора в P2O5:
W ( P ) = 2 • 31 = 62 / 142 = 0,436 или 43,6%
2) массовая доля фосфора в H3PO4:
W ( P ) = 1 • 31 = 31 / 98 = 0,316 или 31,6%
3) массовая доля фосфора в CaHPO4
W ( P ) = 31 / 136 = 0,2279 или 22,79%
Задание 3
Дано:
m ( CaO ) = 30,6 г.
m ( соли ) - ?
Решение:
1) n ( CaO ) m/Mr = 30,6 / 56 = 0,546 моль
2) CaO + H2SO4 —> CaSO4 + H2O
3) Соотношение молей оксида кальция и соли — 1 : 1, то есть количество вещества ( n ) соли будет такое же самое как и в оксиде кальция
4) m ( CaSO4 ) = n • Mr = 0,546 • 136,14 = 74,39 г.
Задание 4
4P + 5O2 —> 2P2O5
P2O5 + 3H2O —> 2H3PO4
2H3PO4 + 6Na —> 2Na3PO4 + 3H2
2Na3PO4 + 3CaCl2 —> 6NaCl + Ca3(PO4)2
2Na + 2H2O —> 2NaOH + H2 ( гидроксид натрия и водород )
2NaOH + H2CO3 —> Na2CO3 + 2H2O
( карбонат натрия и вода )
Na2CO3 + 2HCl —> 2NaCl + H2O + CO2
( хлорид натрия и карбонатная кислота, которая сразу распадается на воду и углекислый газ )
NaCl + HNO3 —> NaNO3 + HCl ( нитрат натрия и соляная кислота )
Задание 2
Для вычисления массовой доли фосфора используем формулу:
W ( элемента ) = n ( количество атомов элемента ) • Ar ( атомную массу элемента )
и делим это на Mr ( молекулярную массу вещества )
1) массовая доля фосфора в P2O5:
W ( P ) = 2 • 31 = 62 / 142 = 0,436 или 43,6%
2) массовая доля фосфора в H3PO4:
W ( P ) = 1 • 31 = 31 / 98 = 0,316 или 31,6%
3) массовая доля фосфора в CaHPO4
W ( P ) = 31 / 136 = 0,2279 или 22,79%
Задание 3
Дано:
m ( CaO ) = 30,6 г.
m ( соли ) - ?
Решение:
1) n ( CaO ) m/Mr = 30,6 / 56 = 0,546 моль
2) CaO + H2SO4 —> CaSO4 + H2O
3) Соотношение молей оксида кальция и соли — 1 : 1, то есть количество вещества ( n ) соли будет такое же самое как и в оксиде кальция
4) m ( CaSO4 ) = n • Mr = 0,546 • 136,14 = 74,39 г.
Задание 4
4P + 5O2 —> 2P2O5
P2O5 + 3H2O —> 2H3PO4
2H3PO4 + 6Na —> 2Na3PO4 + 3H2
2Na3PO4 + 3CaCl2 —> 6NaCl + Ca3(PO4)2
Bangtansonentan:
Пасибо!!!!
Я забыл подписать продукты реакции в последнем задании:
1) Оксид фосфора ( V )
2) Ортофосфатная кислота
3) Ортофосфат натрия и водород
4) Хлорид натрия и ортофосфат кальция
1) Оксид фосфора ( V )
2) Ортофосфатная кислота
3) Ортофосфат натрия и водород
4) Хлорид натрия и ортофосфат кальция
))
пасибо))
Похожие вопросы
Предмет: Русский язык,
автор: xachaturovaler
Предмет: Русский язык,
автор: wwe48
Предмет: Русский язык,
автор: jojo6
Предмет: Английский язык,
автор: Бонни001
Предмет: Химия,
автор: oliachepiga