Предмет: Химия,
автор: ritapyankova
Назовите элемент в атоме которого содержится 13 d-электронов.
В прошлый раз я и ещё 2 человека очень долго сидели и думали над этим.
В итоге: один говорит, что такого нет т. к. провал, другой говорит что технеций, я склонна тоже к технецию, но может быть будет ещё одно мнение со стороны?
Есть схема, но никто не знает насколько она правдоподобна. Помогите
Приложения:
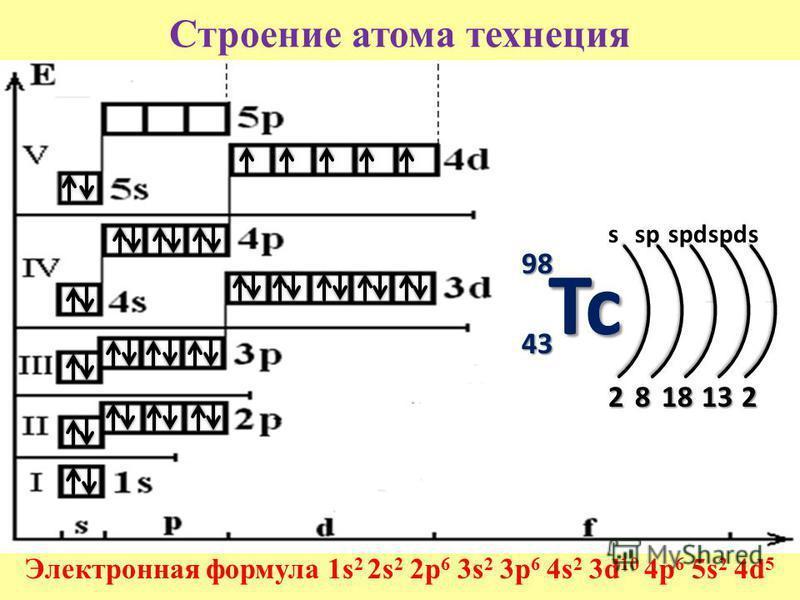
Ответы
Автор ответа:
0
На пяти атомных d - орбиталях может находится только 10 электронов. На орбиталях 3d десять электронов
у атома цинка, у 4d элементов у иттрия 4d1, у циркония 4d2. Значит у циркония есть 3d10 электронов и три электрона на
4d подуровне, всего суммарно 13.
у атома цинка, у 4d элементов у иттрия 4d1, у циркония 4d2. Значит у циркония есть 3d10 электронов и три электрона на
4d подуровне, всего суммарно 13.
Автор ответа:
0
В пределах одной оболочки максимальное число d-электронов равно 10
4-й период пропускаем, там идет заполнение первой d-оболочки.
В 5-м периоде нам нужен элемент, у которого на внешней d-оболочке 3 электрона.
Y: 4d1 5s2; Zr: 4d2 5s2; Nb: 4d4 5s1; Mo: 4d5 5s1;... дальше можно не смотреть.
4-й период пропускаем, там идет заполнение первой d-оболочки.
В 5-м периоде нам нужен элемент, у которого на внешней d-оболочке 3 электрона.
Y: 4d1 5s2; Zr: 4d2 5s2; Nb: 4d4 5s1; Mo: 4d5 5s1;... дальше можно не смотреть.
Автор ответа:
0
что на
Автор ответа:
0
что на это скажете? вот и ломаю голову уже 2 недели.
Автор ответа:
0
Возможно такого элемента не существует. Хотя, может есть какое-нибудь возбужденное состояние, например, ниобия, когда один его d-электрон перескакивает на s-оболочку - и тогда его d-электронов становится ровно 13, но это состояние не является основным.
Похожие вопросы
Предмет: История,
автор: sagdinabahadirova
Предмет: Математика,
автор: 777ina
Предмет: Английский язык,
автор: nurik7627
Предмет: Математика,
автор: alika2005